
The term "corrosion" comes from the Latin word "corrodere", which means to "eat away" anything.
Corrosion is the spontaneous destruction of metals under the influence of chemical or physico-chemical influence of the environment. In a broad sense, not only metals are corroded, but also any materials, be it concrete, plastic, rubber or ceramics.
The cause of the occurrence and course of corrosion processes is the thermodynamic instability of materials to certain components in their environment. Corrosion results in corrosion products (for example, rust), damaged equipment, structural failure.
Every year corrosion causes enormous damage to the national economy of each country. In industrialized countries, the corrosion damage for the year averages about 3-5% of the gross domestic product. And metal loss reaches 20%. The damage from corrosion develops not only from the cost of materials, but also from the costs of manufacturing the damaged structures, equipment and various products.
Corrosion causes both direct and indirect damages.
The indirect losses include losses due to equipment failure, which has become unusable due to corrosion processes, its downtime, replacement or repair, damage to products of other industries due to contamination with corrosion products, high corrosion tolerances, the cost of additional electricity, water, materials and etc.
To direct - the cost of corrosion-damaged pipelines, equipment, machinery, etc.
Corrosion by the mechanism of flow is divided into chemical and electrochemical. More common - the second type.
The science of corrosion and the protection of metals studies the features and mechanisms of the course of corrosion processes in various environments. The task of the science of corrosion is not only the study, but also the development of methods for protecting various materials from corrosion.
The science of corrosion involves not only the knowledge of all the laws of the processes of corrosion. It is also necessary to know well the properties of metals, various materials. In the study of corrosion of metals and methods of protection against it, the scientific base is physical chemistry and metal science.
It is important to know that corrosion is a multistage complex process that needs to be studied holistically. Only by studying the very essence of the corrosion process, you can begin to study and develop methods of protection.
The basis of corrosion processes are redox reactions of metals with the environment, accompanied by the transition of metals to a more thermodynamically stable state.
Consider the corrosion of iron as an electrochemical process. Iron rusting is nothing but an anodic reaction.
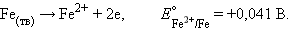
Cathodic reaction - atmospheric oxygen reduction:
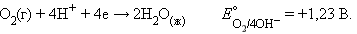
Hydrogen ions are supplied by water. If there was no dissolved oxygen in the water, then corrosion would not be possible. Consequently, iron corrodes in a water layer saturated with oxygen. Thus, the initial stage of iron corrosion can be transferred by reaction
| 2Fe + O2 + 4H + → 2FeO + 2H2O. |
The corrosion rate is significantly affected by the concentration of H + ions. Increasing the pH slows down the corrosion, because the reduction of O2 from H2O slows down. At pH = 9–10, iron corrosion practically stops. It is known that in an aqueous medium, Fe2 + ions in the presence of oxygen are oxidized to Fe3 +. The second stage of corrosion corresponds to the formation of hydrated iron oxide (rust) Fe2O3 ∙ n H2O (Fig. 7.4):
| 4Fe2 + + O2 + 4H2O + x H2O = 2Fe2O3 ∙ x H2O + 8H +. |
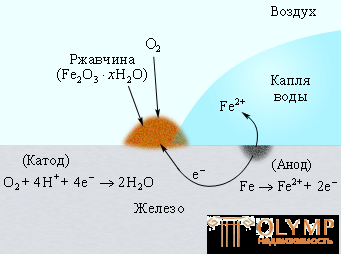 Figure 7.4
Figure 7.4
Corrosion of iron by atmospheric oxygen dissolved in water
To protect iron from corrosion, various coatings are used: paint, a layer of metal (tin, zinc). In this case, the paint and tin protects against corrosion as long as the protective layer is intact. The appearance of cracks and scratches in it contributes to the penetration of moisture and air to the surface of iron, and the corrosion process resumes, and in the case of tin coating it even accelerates, since tin serves as a cathode in the electrochemical process (Fig. 7.5).
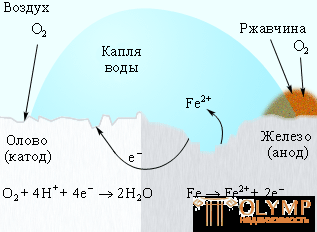 Figure 7.5
Figure 7.5
Corrosion "tinplate"
Galvanized iron behaves differently. Since zinc acts as an anode, its protective function is preserved even if the zinc coating is disturbed (Fig. 7.6).
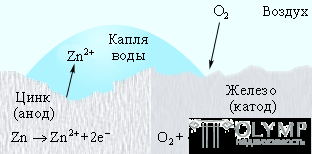 Figure 7.6
Figure 7.6
Cathodic protection in galvanized iron
Cathodic protection is widely used to reduce the corrosion of underground and underwater pipelines and steel supports of high-voltage gears, oil platforms and berths.
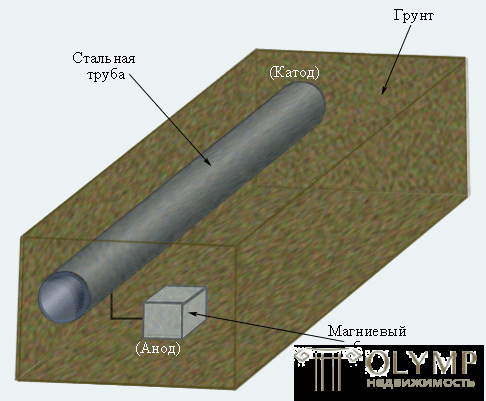 Figure 7.7
Figure 7.7
Underground pipeline cathodic protection
Chemical corrosion is a type of corrosion destruction of a metal associated with the interaction of a metal and a corrosive environment, in which the metal is simultaneously oxidized and the corrosive environment is restored. Chemical corrosion is not associated with the formation, as well as the influence of electric current.
The driving force (root cause) of chemical corrosion is the thermodynamic instability of metals. They can spontaneously move into a more stable state as a result of the process:
Metal + Oxidative Compound = Reaction Product
In this case, the thermodynamic potential of the system decreases.
The sign of changes in thermodynamic potential can determine the possibility of spontaneous chemical corrosion. The criterion is usually the isobaric-isothermal potential G. When the chemical process proceeds spontaneously, the isobaric-isothermal potential decreases. Therefore, if:
Δ GT <0, then the process of chemical corrosion is possible;
Δ GT> 0, then the process of chemical corrosion is impossible;
Δ GT = 0, then the system is in equilibrium.
Chemical corrosion includes :
- gas corrosion - corrosion destruction under the influence of gases at high temperatures;
- corrosion in non-electrolyte fluids.
Gas corrosion is the most common type of chemical corrosion. At high temperatures, the metal surface is destroyed by the action of gases. This phenomenon is mainly observed in metallurgy (equipment for hot rolling, forging, stamping, parts of internal combustion engines, etc.)
The most common case of chemical corrosion is the interaction of metal with oxygen. The process proceeds according to the reaction:
Me + 1/2 O2 - MeO
The direction of this reaction (oxidation) is determined by the partial pressure of oxygen in the gas mixture (pO2) and the pressure of dissociation of oxide vapors at a certain temperature (pMeO).
This chemical reaction can proceed in three ways:
1) pO2 = pMeO, equilibrium reaction;
2) pO2> pMeO, the reaction is shifted towards the formation of oxide;
3) pO2 <pMeO, oxide dissociates into pure metal and oxide, the reaction proceeds in the opposite direction.
Knowing the oxygen partial pressure of the gas mixture and the dissociation pressure of the oxide, it is possible to determine the temperature range at which this reaction is thermodynamically possible.
The rate of flow of gas corrosion is determined by several factors: the ambient temperature, the nature of the metal or alloy composition, the nature of the gas medium, the time of contact with the gas medium, and the properties of the corrosion products.
The process of chemical corrosion largely depends on the nature and properties of the oxide film formed on the surface.
The process of appearance on the surface of the oxide film can be divided into two stages:
- on the surface of the metal, which is in direct contact with the atmosphere, oxygen molecules are adsorbed;
- the metal interacts with the gas to form a chemical compound.
In the first stage, an ionic bond arises between the surface atoms and oxygen: an oxygen atom takes two electrons from the metal. In this case, a very strong bond arises, much stronger than the bond of oxygen with a metal in the oxide. Perhaps this phenomenon is observed due to the effect on the oxygen of the field created by the metal atoms. After complete saturation of the surface with an oxidizing agent, which occurs almost instantaneously, at low temperatures, physical adsorption of oxidant molecules can be observed due to van der Waltz forces.
The result is a very thin monomolecular protective film, which thickens over time, making it difficult for the oxygen to approach.
In the second stage, due to chemical interaction, the oxidizing component of the medium takes the valence electrons from the metal and reacts with it to form a corrosion product.
If the formed oxide film has good protective properties, it will inhibit the further development of the process of chemical corrosion. In addition, the oxide film greatly affects the heat resistance of the metal.
There are three types of films that can form:
- thin (invisible to the naked eye);
- medium (give colors tint);
- thick (well visible).
In order for the oxide film to be protective, it must meet certain requirements: not to have pores, to be solid, to adhere well to the surface, to be chemically inert with respect to its environment, to have high hardness, to be wear-resistant.
If the film is friable and porous, moreover, it still has poor adhesion to the surface - it will not have protective properties.
There is a continuity condition, which is formulated as follows: the molecular volume of the oxide film must be greater than the atomic volume of the metal .
Continuity - the ability of the oxide to cover with a continuous layer the entire surface of the metal.
If this condition is met, then the film is solid and, accordingly, protective.
But there are metals for which the condition of continuity is not an indicator. These include all alkaline, alkaline-earth (except beryllium), even magnesium, which is technically important.
Many methods are used to determine the thickness of the oxide film formed on the surface and to study its protective properties. The protective ability of the film can be determined during its formation, by the oxidation rate of the metal and the nature of the change in velocity over time. If the oxide is already formed, it is advisable to investigate the thickness and its protective properties by applying some suitable reagent to the surface (for example, a solution of Cu (NO3) 2, which is used for iron). By the time of penetration of the reagent to the surface, you can determine the film thickness.
Even the already formed continuous film does not stop its interaction with the metal and the oxidizing medium.
The influence of external and internal factors on the rate of flow of chemical corrosion.
Temperature has a strong influence on the rate of chemical corrosion. With its increase, the oxidation processes go much faster. At the same time, a decrease in the thermodynamic possibility of the reaction does not matter.
Especially strongly influenced by variable heating and cooling. In the protective film due to the appearance of thermal stresses, cracks are formed. Through cracks the oxidizing component of the medium has direct access to the surface. A new oxide film is formed, and the old one is gradually exfoliated.
A major role in the corrosion process is played by the composition of the gas medium. But this is individual for each metal and varies with temperature fluctuations. For example, copper corrodes very quickly in an oxygen atmosphere, but is stable in an environment containing SO2. Nickel, on the contrary, intensively corrodes upon contact with the atmosphere of SO2, but is stable in O2, CO2, and H2O environments. Chromium is relatively stable in all four environments.
If the dissociation pressure of the oxide is higher than the pressure of the oxidizing component - the metal oxidation stops, it becomes thermodynamically stable.
The rate of oxidation depends on the composition of the alloy. Take iron, for example. Additives of sulfur, manganese, phosphorus and nickel do not affect its oxidation. Silicon, chromium, aluminum - slow down the process. And beryllium, cobalt, titanium and copper greatly inhibit oxidation. At high temperatures, tungsten, molybdenum, and also vanadium can intensify the process. This is due to the volatility or low melting point of their oxides.
Observing the oxidation rate of iron at different temperatures, we note that with increasing temperature, the slowest oxidation is observed with an austenitic structure. It is the most heat-resistant compared to others.
The rate of flow of chemical corrosion is also affected by the nature of the surface treatment. If the surface is smooth, then it oxidizes a little slower than a rough surface with defects.
Non-electrolyte fluids are fluids that are not conductors of electricity. These include: organic (benzene, phenol, chloroform, alcohols, kerosene, oil, gasoline); inorganic origin (liquid bromine, molten sulfur, etc.). Pure non-electrolytes do not react with metals, but with the addition of even insignificant amounts of impurities, the interaction process is sharply accelerated. For example, if the oil contains sulfur or sulfur-containing compounds (hydrogen sulfide, mercaptans), the process of chemical corrosion is accelerated. If, in addition, the temperature rises, dissolved oxygen will be in the liquid - chemical corrosion will increase.
The presence of moisture in non-electrolyte fluids provides an intensive course of corrosion by the electrochemical mechanism.
Chemical corrosion in non-electrolyte fluids is divided into several stages:
- oxidizer approach to the metal surface;
- chemisorption of the reagent on the surface;
- the reaction of the oxidizing agent with the metal (formation of the oxide film);
- desorption of oxides with metal (may be absent);
- diffusion of oxides in the nonelectrolyte (may be absent).
To protect structures against chemical corrosion in non-electrolyte fluids, coatings resistant to this environment are applied to its surface.
This section describes how to protect building materials from corrosion in aggressive environments.
Any construction, except for purely force effects, causing a volumetric stress state, is subject to physical and chemical effects of the environment.
The medium can be in a gaseous, liquid or solid form, and most often - multiphase. So, for example, the soil adjacent to them (mainly ground water that saturates it) can act on the foundations; external and internal atmosphere of different humidity and pollution affects walls and coverings.
Certain environmental agents are characterized by greater or lesser aggressiveness with respect to various materials of construction, i.e., the ability to cause complete or partial destruction of them for a certain period of time.
Strictly speaking, every environment affects the structure. These effects can be either aggressive (most often) or favorable, contributing to the stabilization and even hardening of the material.
The main tasks were: assessment of the aggressiveness of different environments, description of the environment on materials and structures, drawing up recommendations on the choice of materials resistant to these environments; The main attention is paid to the effects of chemical agents and the protection of materials and structures from them.
The task of the designer, who assigns this or that material for use in constructions with the presence of aggressive media, is responsible and rather complicated. The use of relatively economical, but often insufficiently resistant materials leads to their rapid destruction and high repair and restoration costs; the use of highly resistant, but usually more expensive materials increases the cost. construction of structures.
The ideal solution, apparently, would be the design of equal-resistant elements of structures that would collapse at the same time and only after a long (specified) service life corresponding to the obsolescence of equipment. However, in practice this is not always possible.Therefore, during the operation of buildings and structures, unfortunately, it is quite often necessary to replace fast-collapsing elements or individual structures — usually floors, less often foundations, walls, coatings, and others. In such cases, when designing a major overhaul at individual enterprises, it is desirable to provide for the possibility of sectional replacement of structures: this will speed up the repair and construction work and allow them to be performed in the conditions of the existing enterprise without disrupting the general process flow.
The medium acts primarily on the surface layers of structures, gradually penetrating into the depths, especially if the material is not sufficiently dense. Therefore, very often the protection of structures against aggressive environmental influences is reduced to the compaction of the material in the surface layer or the application of sufficiently dense and resistant to this environment protective coatings - facings, plasters, adhesive insulation or even paint. Naturally, during the operation of buildings and structures, these protective coatings should be periodically and timely updated.
Considering these circumstances, the mechanism of corrosion processes is considered on the site in relation to the individual materials from which the structures are made - metal, concrete, wood - or materials that are applied to the structure as protective coatings - paints, plastics, mastics and solutions.
Значительное место занимает описание приемов повышения плотности и стойкости обычного цементного бетона, что вполне закономерно: он используется на строительстве; цементные бетоны высокой плотности являются достаточно экономичными и стойкими конструктивными и защитными (для металла) материалами во многих средах, за исключением сильнокислых вод.
Для повышения стойкости материалов и конструкций, работающих в кислых средах, рекомендуются специальные мастики, растворы и бетоны на жидком стекле, серном цементе и битуме.
При наличии переменных (кислото-щелочных) воздействий либо при воздействии высококонцентрированных или подогретых химических растворов необходимо применение более стойких материалов — на основе пластмасс и пластбетонов.
Для первичной защиты строительных конструкций от коррозии используют коррозионно-стойкие для данной среды покрытия. При необходимости предусматривают вторичную защиту поверхности конструкции:
• лакокрасочными покрытиями;
• оклеечной изоляцией из листовых и пленочных материалов;
• облицовкой, футеровкой, применением изделий из керамики, шлакоситалла, стекла, каменного литья, природного камня;
• штукатурными покрытиями на основе цемента, полимерных вяжущих, жидкого стекла, битума;
• уплотняющей пропиткой химически стойкими материалами.
По степени воздействия на строительные конструкции среды разделяются на неагрессивные, слабоагрессивные, среднеагрессивные и сильноагрессивные. По физическому состоянию среды подразделяют на газообразные, твердые и жидкие, а по характеру воздействия на материал конструкции — на химически и биологически активные.
Для бетонных и железобетонных конструкций, предназначенных для эксплуатации в агрессивной среде, при их проектировании коррозионную стойкость обеспечивают применением коррозионно-стойких составляющих, добавок, повышающих коррозионную стойкость самого бетона и его защитную способность для стальной арматуры. В изготовляемых конструкциях должны быть снижены проницаемость бетона, трещиностойкость, ширина расчетного раскрытия трещин и толщина защитного слоя бетона.
The structure of structures should not additionally provide for their protection:
• paint coatings (aerosols) - under the action of gaseous and solid media;
• paint mastic multilayer coatings - with a solid corrosive environment;
• Coating coatings;
• Restoration of the gummed coating;
• sealing materials with chemically resistant materials;
• hydrophobic - with occasional moistening with water or precipitation, the formation of condensate, as a primer under the paintwork.
Measures of protection of reinforced concrete structures from corrosion are assigned in the project of production of works, taking into account the type and features of the protected structures, their manufacturing technology, construction and operating conditions.
For concrete and reinforced concrete structures of buildings and structures with aggressive media, it is necessary to provide for the use of only the following cements: portland cement, slag portland cement, sulphate-resistant, aluminous and tense cements. Chlorine salts are not allowed to be added to concrete for reinforced concrete structures, as well as to solutions for injecting canals, cementing joints and structural joints.
The thickness of the protective layer of concrete for planar structures may be applied equal to 15 mm for slightly aggressive and moderately aggressive media and equal to 20 mm for strongly aggressive media. For similar monolithic structures, the required thickness of the protective layer is increased by 5 mm.
Embedded parts and connecting elements in the joints of structures exposed to a liquid medium must be protected by metallic or combined coatings. On the surface embedded parts must be applied without fail paintwork.
The thickness of the metallization coatings and the metallization layer in the combined coatings should be for zinc and aluminum coatings of at least 120 microns. The hot dip galvanized zinc coatings must be at least 50 microns thick, and by electroplating not less than 30 microns.
To protect wooden structures from corrosion caused by exposure to biological agents, apply antiseptic, canning, coating with paints and varnishes or surface impregnation of complex action. If the design turns out to be in a chemically aggressive environment, then for a protective coating, paintwork materials or impregnation with compounds of complex action.
Depending on the degree of aggressive action, wooden structures are protected with water-soluble and hardly washable antiseptics or by surface treatment with antiseptic pastes. Protective coatings are made of moisture-resistant paints and varnishes or moisture-impregnating impregnating compositions.
For wood protective coatings, lacquers and enamels pentaphthalic, perchlorovinyl, epoxy, epoxy-phenolic, etc. are applicable. Antiseptic is recommended to be performed with sodium fluoride, ammonium fluorofluoride, specially designed for antiseptic preparations. When preserving wood, coal, anthracene and shale oil are recognized as the best drugs.
For chemical protection of wooden structures, chemically resistant, moisture-resistant paints and varnishes and chemically resistant moisture-resistant impregnating compounds have been developed.
Stone and asbestos-cement structures . Aggressive effects on structures of these materials can be gaseous, liquid. In case of saline soils and corrosive liquids, the use of silicate brick constructions, as well as mortars using clay and ash is not allowed. With periodic moistening with an aggressive environment and freezing of masonry, the brand of frost resistance brick should be taken not lower than F50. In case of highly aggressive exposure to acidic media, acid-resistant solutions based on liquid glass or polymeric binders should be used for masonry.
The surfaces of stone and reinforced stone structures should be additionally protected against corrosion: by plaster with a paint-and-lacquer coating, directly by masonry with multi-layer mastic materials.
Asbestos cement wall panels should not come into contact with the ground. These structures must be placed on the basement, which has a waterproofing gasket that protects the panels from capillary suction of corrosive groundwater. The surface of asbestos cement structures should be protected from aggressive media exposure with paint and varnish coatings, the same as for concrete structures.
Metal structures should be coated with anti-corrosion coatings with aggressive media exposure - the atmosphere of air, liquid organic and inorganic media, soils.
Aluminum bearing structures should be protected from corrosion by electrochemical anodization (layer thickness> 15 μm). When operating structures in water, they must be additionally painted with waterproof paintwork materials.
Adjoining of aluminum structures to brick or concrete structures is allowed only after complete hardening of the mortar or concrete, regardless of the degree of aggressive environmental exposure. The junction areas should be protected by paintwork. Concreting aluminum structures is not allowed. Adjoining of painted aluminum constructions to wooden constructions is allowed, provided the latter is impregnated with creosote.
To protect steel and aluminum structures from corrosion, paints and varnishes (primers, paints, enamels, varnishes) are used, which are divided into four groups depending on the degree of aggressive influence of the environment:
I - pentaphthalic, glyptal, epoxy ether, alkyd-styrene, oil, oil-bitumen, alkyd-urethane, nitrocellulose;
II - phenol-formaldehyde, chlorinated rubber, perchlorovinyl, polyvinyl butyral, polyacrylic, acrylic, silicone, polyether silicone, shalevinyl;
III — epoxy, silicone, perchlorovinyl, slate vinyl, polystyrene, polyurethane, phenol formaldehyde;
IV — perchlorovinyl and epoxy.
Hot-dip galvanizing and melt-dip aluminization should be provided for corrosion protection of steel structures with bolted joints, as well as bolts, nuts and washers. Thermal spraying of zinc and aluminum should be provided for the protection of steel structures with welded, bolted and riveted joints. Electrochemical protection is mandatory for steel structures that are immersed in the ground or in inorganic liquid media, the inner surfaces of the bottoms of tanks for petroleum and petroleum products.
Chemical oxidation with subsequent painting or electrochemical anodizing of the surface should be provided to protect aluminum structures from corrosion. Structures where the integrity of the protective anodic or lacquer film is compromised during welding, riveting and other processes performed during installation should be cleaned after preliminary cleaning. protected by paintwork using tread primer.
To prevent corrosion of buildings and structures, various methods of protection are used, including metallization, painting with paint and varnish compounds, gumming and water repellency.
Metallization is used to protect metal and embedded parts of reinforced concrete structures. Use zinc or aluminum wire, the layer thickness of the applied protective coating 0.2 ... 0.5 mm.
The paintwork is used to protect metal structures from corrosion. Apply oil paints, varnishes, enamels based on synthetic resins, bituminous mastics and solutions. The protective coating consists of a primer and coating layers, the amount of which depends on the purpose of the coating, the properties of the protected material, the technological conditions of the process of applying and operating the coating.
The primer is applied on a cleaned and dry surface, it should not have gaps, smudges and other defects on the painted surface, so it is applied in thin layers (preferably at least two). On the basis prepared by a primer put the main layers of coloring. The number of layers is determined depending on the purpose of the coating, the application process, the properties of the material to be protected and the operating conditions of the coating. Coating with several layers minimizes penetration of the corrosive medium through possible pores of one or even two layers. Coating with one layer of large thickness leads, as a rule, to the appearance of cracks, disruption of the integrity of the coating and poor adhesion (adhesion) to the base. In multilayer coating, each subsequent layer is applied after complete drying and curing of the previous one.
Coloring is done by mechanized and manual methods. In the mechanized method using pneumatic or mechanical sprayers. When painting small forms, lattice structures, in hard-to-reach places, manual painting is preferable to avoid large losses of paints and varnishes.
Gumming - drawing on a surface of crude rubber with the subsequent curing. A thin layer of rubber glue is applied to the de-watered surface, cleaned from dirt and dust, and then applied onto sheet rubber or rolled rubber and subjected to temperature treatment — vulcanization. The result is a continuous protective coating thickness, depending on the thickness of the raw rubber (2..A mm). It is allowed to apply on the surface several layers of a solution of raw rubber in gasoline. Layers are applied 40 ... 60 minutes after drying the previous one, then the coating is vulcanized.
Water repellency - coating the surfaces of reinforced concrete and masonry structures with aqueous solutions of organosilicon compounds. On the surface, covered with the composition, a protective waterproof film is formed that prevents water penetration and corrosion of materials. The application of the solutions carried out with brushes, rollers, spray guns, other means of small-scale mechanization. Coverage is 3 ... 5 years, it must be periodically updated.
Anticorrosion coating is performed at positive temperatures. If it is necessary to work at negative temperatures, it is necessary to heat the base, use heated compositions, and provide thermal protection for the coatings.
When choosing and arranging anti-corrosion coatings, the requirements of SNiP 3.04.03-85 should be followed. Protection of building structures and facilities from corrosion, SNiP 2.03.11-85. Corrosion protection of building structures.

Что бы оставить комментарий войдите
Комментарии (0)